Veeva Vault EDC
Run the Study You Need
Electronic Data Capture that handles the
needs of today’s complex trials.
Announced 2016 Status Mature Customers 100+
Eight of the top 20 biopharmas standardize on Vault EDC to build a modern clinical data foundation
Overview
Run studies 50% faster
with 50% less effort
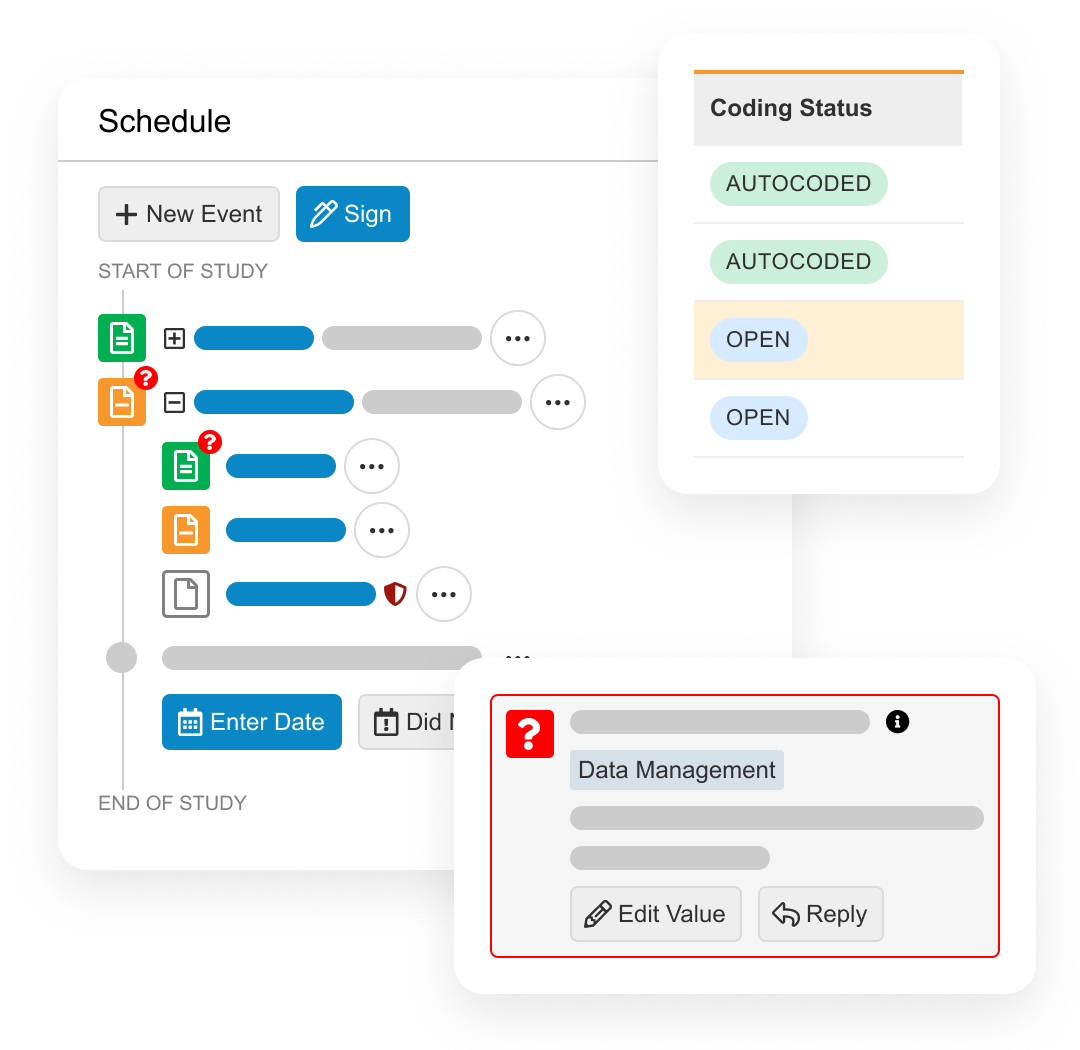
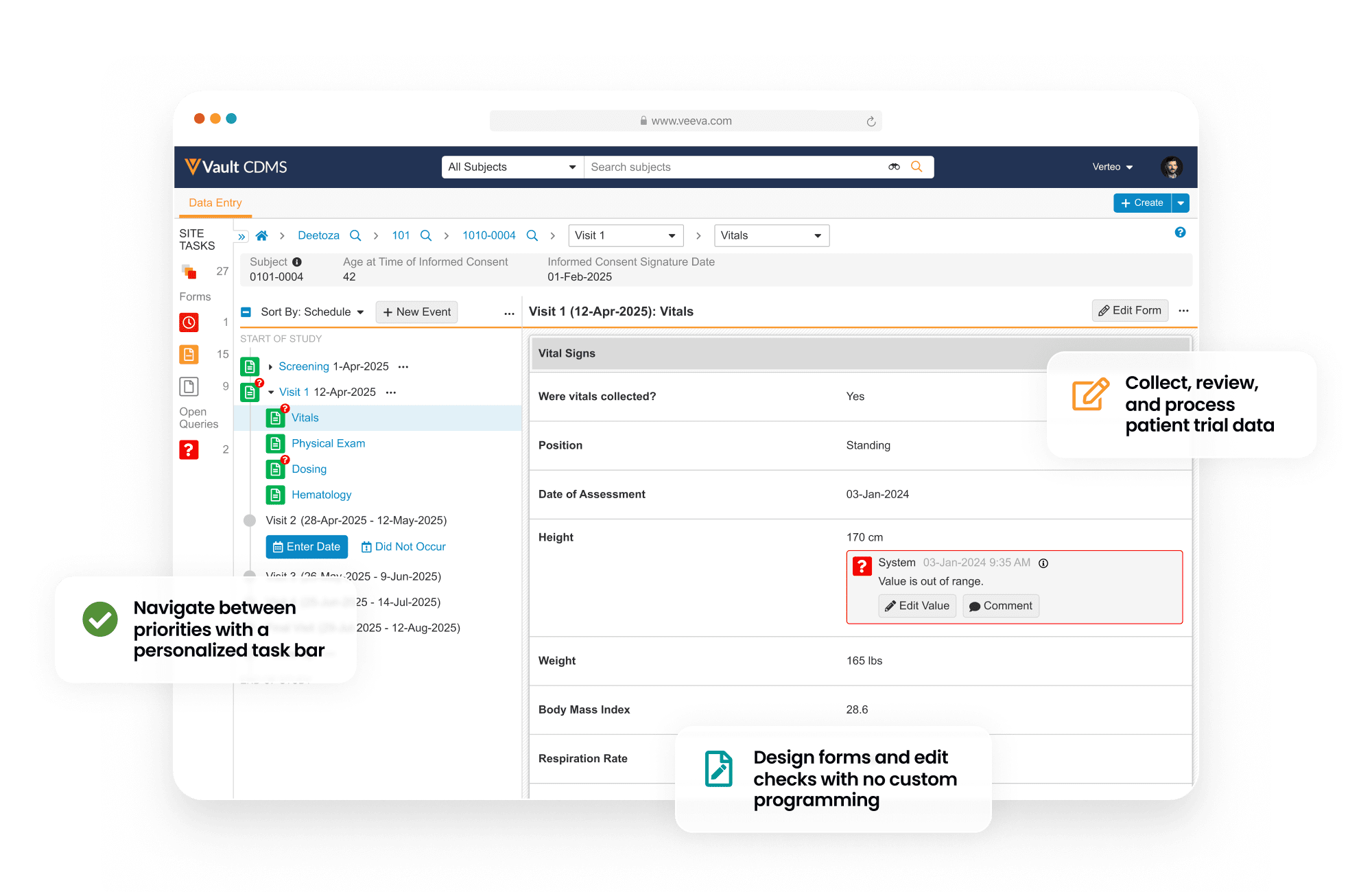
Vault Electronic Data Capture (EDC) provides an end-to-end environment to collect, review, and process trial data about patients.
During study start, Vault EDC is used to design patient forms (including edit checks) without the need for custom programming.
During study execution, Vault EDC collects all patient form data, local labs, and medical coding. It also has quality controls including querying, targeted source data verification (SDV), and protocol deviations. When protocol amendments happen, the Vault EDC database needs no downtime.
At the end of the study, Vault EDC provides data lock and post-processing features, including end-of-study media creation and archiving.
Impact
Proven value for complex studies
50%
faster study builds
100%
elimination of known custom functions
9x
faster to implement study changes
Why Vault EDC
Modern EDC for agility and efficiency
Customer Success
Eight of the Top 20 biopharmas
switched to Vault EDC